July
2020
HYDROCARBON
ENGINEERING
26
they wore away. The subsequent fouling was then as bad as,
or worse than, before the coating was applied. The wear
resistance of the coating was evaluated using a
Taber Abraser per ASTM D4060. In this test, the specimen is
rotated as an abrasive laden rubber wheel rolls against the
surface. The weight loss of the specimen was measured
with cycles and plotted (Figure 3). By a wide margin,
Pos-E-Coat was the least durable coating tested.
Pos-E-Coat Plus faired rather well, but showed more weight
loss than Coating 18. While Pos-E-Coat 523 had similar
weight loss results to Pos-E-Coat Plus, these values do not
take density into account. This coating is denser by a factor
of 5 or more than the rest of the coatings, so the actual
material lost during the test was much less.
Corrosion resistance
The final key area evaluated was corrosion resistance, or
the ability of the coating to act as a protective barrier. This
is of vital importance in hydrogen recycle service since, as
mentioned above, the salt deposits are very corrosive.
Coating suppliers typically use a salt spray test to evaluate
barrier properties. In
this case, the use of a
salt spray is
applicable, but in
general, it does not
make sense for most
applications. The
results of a salt spray
test are also very
subjective. For this
reason,
electrochemical
impedance
spectroscopy (EIS) was
used for the
evaluation. This
method can replicate
the relative ranking of
a salt spray test in a
fraction of the time
and gives several advantages, such as the
ability to use more relative electrolytes,
providing quantitative results, and giving some
insight into the corrosion processes at work.
The electrolyte used in these tests was a
saturated ammonium chloride solution.
Ammonium chloride is typically the main salt
found in the foulant in hydrogen recycle
compressors. Tests were performed at various
times over a 500 to 2000 hour exposure
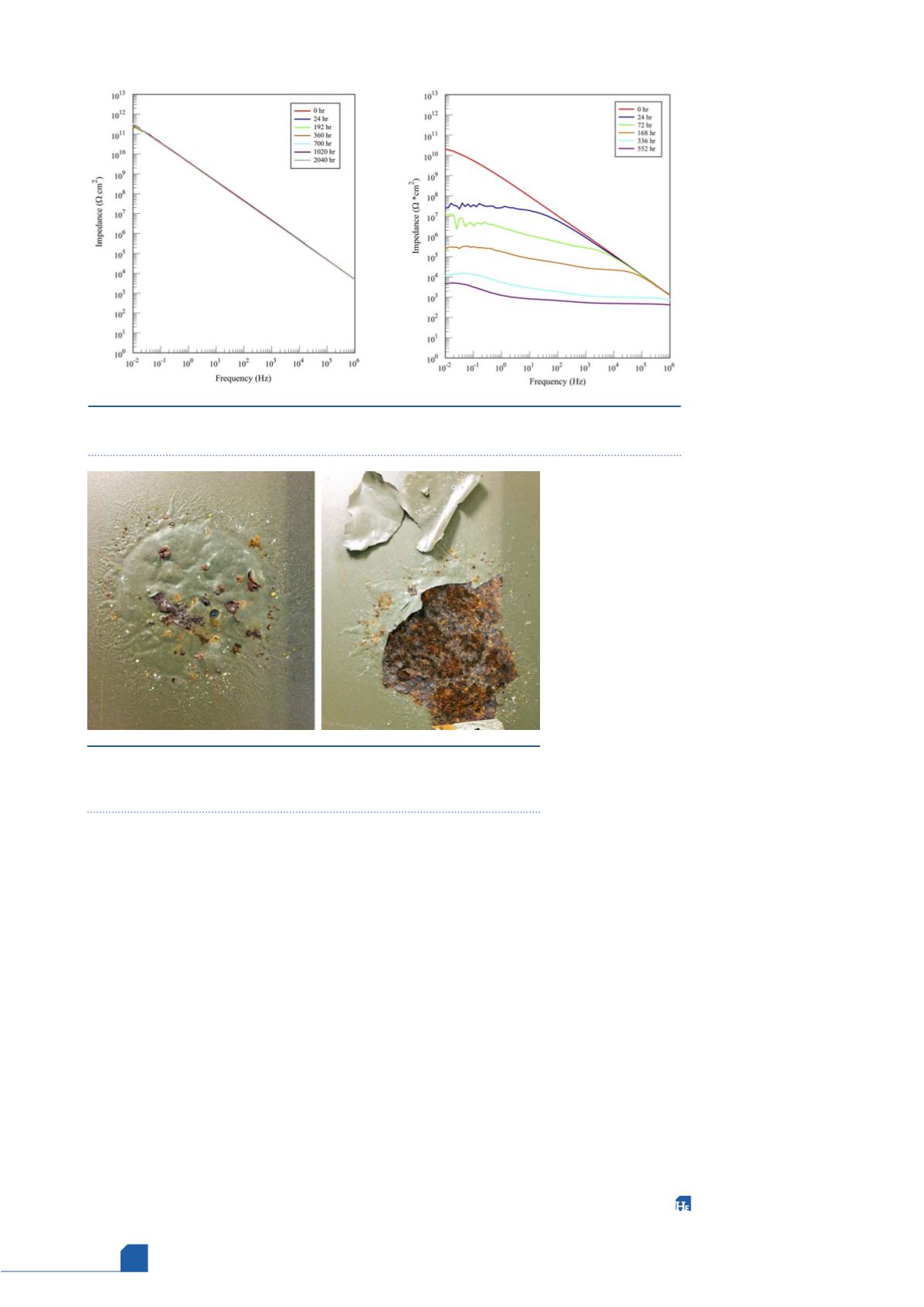
period. After 2000 hours, Coating 18 and
Pos-E-Coat Plus had little to no change from
the values at the beginning of the test. They
acted as perfect barrier coatings, as shown in
Figure 4 (left). Pos-E-Coat behaved similarly
over that period, but started to show signs of
being permeated by the electrolyte.
Pos-E-Coat 523 showed some corrosion in the
results, but was found to be cracked after the
test. Another interesting result from some of the other
coatings that were rapidly permeated was that the
aluminium in the base coating was aggressively attacked by
the electrolyte. Figure 4 (right) shows the EIS results from a
coating that behaved this way, and Figure 5 shows the
exposed area after the test was completed. This indicates
that the use of the aluminium filled cermet base coating is
probably not a good idea in a hydrogen recycle application.
Conclusion
At the end of the project, it was easy to conclude that
Coating 18 was superior to the others in a hydrogen
recycle application. It had the best fouling resistance by a
large margin, was one of the top performers in the wear
testing, and edged out Pos-E-Coat Plus in the corrosion
tests. It also did not utilise the aluminium containing
bond coating in most of the other coatings tested. It is
also very clear that while the ‘one size fits all’ approach
has been successful, significant gains in coating
performance can be achieved by using a targeted
approach to coating selection.
Figure 5.
Exposed area of a permeated coating immediately after
test (left) and after peeling back the coating to reveal the substrate
(right).
Figure 4.
EIS test results in saturated ammonium chloride electrolyte for Coating 18 (left)
and a coating that was permeated (right).








